Benign Pleural Effusions
Lateral collection
(The text is taken from the doctoral thesis of Dr Athanasios Kleontas)
In humans, under normal conditions, the pleural cavity contains 0.26±0.1 ml/kg of pleural fluid, i.e. approximately 20 ml (10).
Pathogenesis of pleural effusion
Pleural effusion will occur when the rate of pleural fluid production exceeds the rate of fluid absorption. The main mechanisms leading to pleural fluid accumulation in the pleural cavity are (48):
- the changes in the equilibrium of the component pressures
- disorders in lymphatic drainage, and
- changes in the permeability of the capillary microcirculation and mesothelial cells
The main factors that lead to an increase in production and decrease in fluid absorption are:
- Increase production of the liquid
Increased production of interstitial fluid in the lung
Left heart failure
Pneumonia
Pulmonary embolism
Increase in intravascular pressure in the pleura
Right or left heart failure
Superior vena cava syndrome
Increase in pleural fluid protein
Reduction of pleural pressure
Atelectasis of the lung
Increase in the elastic recoil of the lung
Increase in fluid in the peritoneal cavity
Ascetic
Peritoneal dialysis
Erosion of the major thoracic duct
- Reduction of the absorption of pleural fluid
Obstruction of the lymphatic vessels in the pleural wall of the pleura
Increase in venous pressure
Superior vena cava syndrome
Right heart failure
The most common causes of pleural effusion are (64):
- Heart failure with reduced ejection fraction or acute heart failure
- Bacterial pneumonia
- Pulmonary embolism
- Neoplasia
- Viral infections
- Cardiac surgeries
Causes of pleural effusion
The causes of benign pleural effusion are categorized as follows:
Infectious causes
- Bacterial infections
- Bacterial pneumonia
- Parapneumonic collection and inspiration
- Tuberculosis
- Viral infections
- Viral pneumonias
- Parapneumonic collection and inspiration
- HIV
Neoplastic causes
- Kaposi's sarcoma
- Lymphoma (chylothorax, pyothorax)
- Mesothelioma
- Ovarian cancer
- Meigs syndrome
Lung diseases
- Trapped lung
- Pulmonary embolism
- Pulmonary sarcoidosis
- Exposure to asbestos
Cardiological diseases
- Heart failure
- Compressive pericarditis
- Pulmonary vein stenosis
- Superior vena cava syndrome
- Dressler's syndrome
Liver diseases
- Alcoholism
- Cirrhosis
- Amoebic liver abscess
Gastrointestinal diseases
- Intra-abdominal abscess
- Esophageal perforation
- Chronic pancreatitis
- Acute pancreatitis
- Postoperative period of abdominal surgery
Autoimmune diseases
- Rheumatoid arthritis
- Systemic lupus erythematosus
- Pharmacogenic seronitis
- Sjogren's syndrome
- Wegener's carcinoma
- Churg-Strauss syndrome
Diseases of the reproductive system
- Endometriosis
- Ovarian hyperstimulation syndrome
- Urinothorax
Other causes
- Idiopath
- Trauma
- Iatrogenic injury
- Peritoneal dialysis
- Hypothyroidism
- Uremic pleurisy
- Pseudostraw
- Ascetic
- Yellow eye syndrome
- Coronary heart surgery
Medicines
- Amiodarone
- Beta blockers
- Alkaloids of the erysipelas
- L-tryptophan
- Methotrexate
- Nitrofurantoin
- Phenytoin
- Praktololi
- Minoxidil
- Methysergides
- Bromocriptine
- Blueomycin
- Mitomycin
- Procarbazine
- Cyclophosphamide
- Interleukin-2
- Dasatinibis
- Dandrolene
In particular in the case of chylothorax, the causes are (65):
- Malignant obstruction of the thoracic duct
- Trauma
- Iatrogenic injuries
- Genetic causes
- Thoracic duct atresia
- Communication of the thoracic duct with the pleural cavity
- Trauma during childbirth
- Other causes
- Lymphangioleiomyomatosis
- Gorham's disease
- Noonan syndrome
Correlation between etiological factor of pleural effusion and clinical picture
Depending on the etiopathogenesis of pleural effusion, the clinical findings, as obtained during history taking and clinical examination of the patient, are also relevant (64):
- Heart failure
Hypoxygenemia
Pulmonary or peripheral edema
Jugular distension
3ος heart rate (S3 gallop)
- Bacterial pneumonia
Cough
Fever
Quivering
- Pulmonary embolism
Shortness of breath
Chest pain
Quivering
Unilateral swelling of the lower limb
Immobilisation or recent long journey
Obesity or pregnancy
- Malignancy
History of malignancy
Tumour of lung, breast, pancreas, colon
Fever
- Viral infection
Cough
Easy fatigue
Myalgia
Fever
Exanthema
- Pericarditis
Acute chest pain
Jugular distension
Pericardial collection
Electrocardiographic changes
Pulmonary vein stenosis
Recent cardiac catheterization
Pulmonary hypertension
- Superior vena cava syndrome
Facial swelling
Swelling of the upper limbs
Thoracic epiphlebitis
- Intra-abdominal abscess
Abdominal pain
Quivering
Nausea
Vomiting
Fever
- Cirrhosis of the liver
Ascetic
Medusa head (abdominal epiglottis)
Palmar erythema
History of alcoholism or viral hepatitis
- Esophageal perforation
Chest or abdominal pain
Fever
Esophageal volume
Gastroesophageal reflux disease
Recent upper digestive endoscopy
- Pancreatitis
Abdominal pain
Nausea
Vomiting
Anorexia
Increased levels of amylase and lipase
- Endometriosis
Pelvic pain
Infertility
Dysmenorrhea
- Meigs syndrome
History of ovarian cancer
Ovarian hyperstimulation syndrome
Infertility treatment history
Abdominal pain
- Urinothorax
Urinary obstruction
Recent urological surgery
- Mesothelioma
Pleural volume
History of exposure to asbestos
- Chylothorax
Chest volume
Chest injury
Lipids in the pleural fluid
Pseudostraw
- Rheumatoid disease
- History of tuberculosis or pleurisy
- Nephrotic syndrome
Swelling
Proteinuria
- Rheumatoid arthritis
Swelling and joint pain
- Yellow eye syndrome
Lymphedema
Yellow nails
- Bronchiectasis
Diagnostic approach to pleural effusion
In summary, the initial diagnostic approach to suspected pleural effusion involves the following steps (66):
- Chest radiograph (supine, anteroposterior, lateral, lateral supine) and imaging confirmation of pleural effusion (Figure 10A)
- Diagnostic thoracocentesis (best performed under echo-guided thoracic ultrasound - echo-guided thoracocentesis)
- Pleural fluid analysis
- Cytological examination
- Blood test
- Haematocrit
- White blood cell count and type
- Biochemical test
- pH
- Glucose
- Lactic dehydrogenase (LDH)
- Albumin
- Total proteins
- In special cases:
- Adenosine diaminase (ADA)
- Cholesterol
- Triglycerides
- B-type natriuretic peptide (BNP)
- Urias
- Creatinine
- Amylase
- Cancer markers
- Microbiological examination
- Cultivation for pathogens and fungi
- Direct Gram staining
- Calculation of Light criteria to differentiate whether the liquid is a di- or exudate
- Pleural fluid protein/serum protein ratio > 0.5
- Pleural fluid LDH/serum LDH ratio > 0.6
- Pleural fluid LDH > 2/3 of the upper normal value of blood serum LDH
- Transthoracic ultrasound for the detection of diaphragms, inclusions and masses
- Chest CT in complicated parapneumonic collections (Figure 10B, 10C)
- Percutaneous biopsy or better thoracoscopy for drainage and taking biopsies
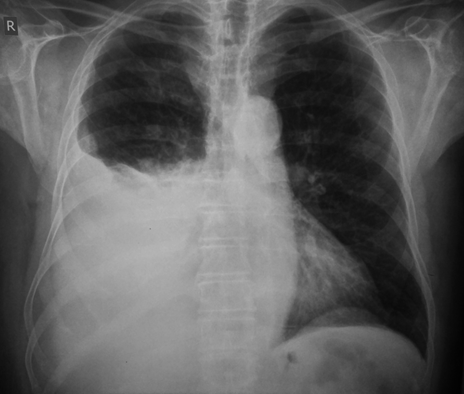
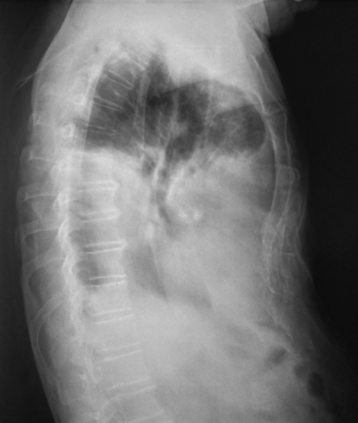
Chest X-rays with pleural effusion of the right hemithorax
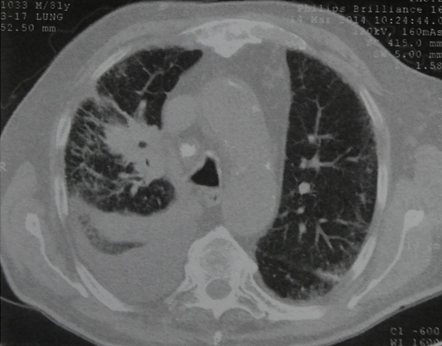
Computed tomography of the chest with pleural effusion of the right hemithorax
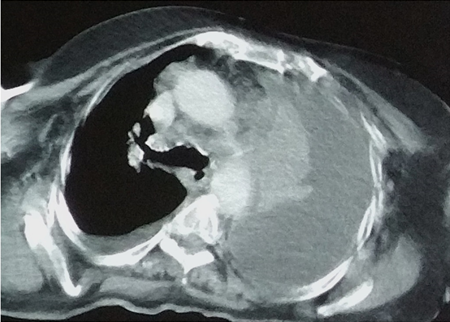
Computed tomography of the chest with pleural effusion of the left hemithorax
Diagnostic assessment of pleural fluid markers
Macroscopic examination of pleural fluid
The macroscopic characteristics of pleural fluid, such as colour, complexion, clarity, consistency and odour can help us in the differential diagnosis of its aetiology (66). Thus, in the case of haemorrhagic pleural fluid, whose haematocrit is > 50% of the haematocrit of the patient's peripheral blood, we are tipped off that it is haemothorax. In general, bleeding pleural fluid is seen in cases of malignancy, trauma, tuberculosis, pulmonary embolism with infarct, postoperative, ruptured arteriovenous malformations (telangiectasias) and catamenial pneumothorax. In the case of milky complexion, there is a strong suspicion of chylothorax or pseudochylothorax. In the case of fever caused by anaerobic microbes, the pleural fluid is cloudy, purulent and foul-smelling. When food debris is found in the pleural fluid, the possibility of rupture of the oesophagus is high. If the pleural fluid is yellowish-green (like bile), there is a possibility of cholothorax. When the pleural fluid looks like anchovy sauce, it is a rupture of an amoebic abscess. If the pleural fluid has an ammonia smell, it is probably a urinothorax. Black pleural fluid has been reported in cases of malignant melanoma, Aspergillus niger and Rhizopus oryzae infection and rupture of the pancreatic cyst (67).
Haematological examination of pleural fluid
This test essentially determines the red blood cell count and the number and type of white blood cells (68):
- When red blood cells (RBC) > 100000/mL (100 × 106/L), then the primary disease may be:
- Malignancy
- Trauma
- Parapneumonic collection
- Pulmonary embolism
- Hereditary haemorrhagic telangiectasia
- Catamenial pneumothorax
- When the white blood cell count (WBC) > 10000/mm3 (10 × 109/L), then the likelihood of fever increases
- Neutrophils > 50% of white blood cells recommend:
- Parapneumonic collection
- Empyema
- Pulmonary embolism
- Malignant pleural effusion (20% of cases)
- Acute tuberculous pleurisy (7% of cases)
- Intra-abdominal diseases
- Lymphocytes make up 80% of white blood cells:
- Tuberculosis
- Lymphoma
- Chronic rheumatic pleurisy
- Sarcoidosis
- Late pleurisy after cardiac surgery
Cytological examination of pleural fluid
Cytological examination of pleural fluid can help diagnose malignant neoplasms in 60% of cases. Usually no more than two samples are needed. Further study of the samples by immunohistochemistry can establish the exact identification of the neoplasia. The presence of non-leukocytic cells in the pleural fluid usually indicates the presence of malignancy. The presence of mesothelial cells in the process of mitosis may mimic adenocarcinoma. Usually the results obtained by the cytology laboratory have the following characteristics (66):
- Insufficient sample - absence of mesothelial cells or only degenerated cells
- Satisfactory sample, without identification of neoplastic cells (this does not exclude the existence of malignancy)
- Presence of atypical cells - presence of inflammatory or neoplastic cells
- Suspicious for malignancy sample - cells with malignant characteristics observed
- Malignant pleural effusion - definite identification of neoplastic cells (immunohistochemistry required)
Biochemical examination of pleural fluid
The biochemical examination of the pleural fluid first helps to characterise the pleural fluid as a di- or exudate and then, by detecting specific substances, the differential diagnosis of the primary disease is made. This is followed by an analysis of the most important substances identified qualitatively and quantitatively in pleural fluid (68):
Proteins
- When the protein level is > 3 g/dL (30 g/L), it is an exudate (an insufficient criterion in itself) (68)
- Pleural fluid protein/serum protein ratio > 0.5, recommends exudative collection (68)
- In patients with heart failure receiving diuretic treatment, if the difference between serum and pleural fluid proteins is greater than 3.1 g/dL (31 g/L), it is a diathromy (68)
Galactic dehydrogenase (LDH)
- If LDH > 2/3 of the upper normal value of serum LDH, then it is an exudate (68)
- If pleural fluid LDH/serum LDH ratio > 0.6, recommends exudative collection (68)
- LDH is elevated (usually > 500 units/L) in 75% of patients with tuberculous pleurisy (69)
- Very high LDH levels (usually > 1000 units/L) are detected in complicated parapneumonic collections and tuberculous pleurisy (68)
Glucose
- Glucose < 60 mg/dL (3.3 mmol/L) is seen in complicated parapneumonic effusion, tuberculosis, neoplasia and rheumatoid arthritis (68)
- Glucose < 29 mg/dL (1.6 mmol/L) is seen in rheumatoid arthritis (66)
- Pleural fluid that shows low glucose levels usually also shows low pH and high LDH levels (68)
Adenosine diaminase (ADA)
- ADA > 40 units/L (667 nanocatal/L [nkat/L]) indicates (70):
- Tuberculosis (90% of cases)
- Embolism (90% of cases)
- Complicated parapneumonic effusion (30% of cases)
- Neoplasia (5% of cases)
- Rheumatoid arthritis
pH
- pH < 2 recommends drainage of the collection (66)
- pH < 3 is observed in (66):
- Malignant pleural effusion
- Complicated pleural infection
- Disease of connective tissue (e.g. rheumatoid arthritis)
- Tuberculous pleurisy
- Esophageal perforation
Nucleic acid enhancement (NAA)
- NAA can confirm tuberculous pleurisy with (71):
- Sensitivity 62%
- Specialty 98%
Interferon c
- Gamma interferon appears to have moderate sensitivity (48-88%) and specificity (82%) for the diagnosis of extrapulmonary tuberculosis (72)
Amylase
- Abnormal amylase values are observed in (73):
- Neoplasms (mainly adenocarcinoma), usually sialate amylase
- Acute pancreatitis
- Pseudocyst of the pancreas
- Esophageal perforation, usually salivary amylase
- Rupture of an ectopic pregnancy
- It is not a routine test when there is no clinical suspicion of pancreatitis or ruptured oesophagus
Cholesterol and triglycerides
- In the case of chylothorax (66):
- The presence of chylomicrons confirms chylothorax
- Triglycerides > 110 mg/dL (1.24 mmol/L) confirm chylothorax
- Triglycerides < 50 mg/dL (0.56 mmol/L) exclude chylothorax
- Cholesterol levels are low and no cholesterol crystals are detected
- In the case of pseudochylothorax (66):
- Cholesterol > 200 mg/dL (5.18 mmol/L) confirms pseudochylothorax
- The presence of cholesterol crystals confirms pseudochylothorax
- Usually no chylomicrons are detected
Creatinine
- Bidiremia with pleural fluid creatinine/serum creatinine ratio > 1, and low pH is seen in case of urinothorax (66)
N-terminal pro-B-type natriuretic peptide (NT-proBNP)
- NT-proBNP > 1500 pg/mL observed in heart failure
Cancer markers
- May be useful in the differential diagnosis of malignant from benign pleural effusions
- The sensitivity and specificity of each indicator shall be reported (74)(75)(76)(77)(78)(79):
- CEA: sensitivity 54.9% and specificity 96.2%
- CA3: sensitivity 50.7% and specificity 98.3%
- CA9: sensitivity 25-37.6% and specificity 96-98%
- CA 125: sensitivity 48% and specificity 85%
- CYFRA1: sensitivity 55-62.5% and specificity 91-93.2%
- VEGF: sensitivity 75% and specificity 72%
- Telomerase: sensitivity 76% and specificity 87%
- Survivin: sensitivity 74% and specificity 85%
- Mesothelin: sensitivity 19-68% and specificity 88-100%
Albumin
- The difference between serum blood albumin minus pleural fluid albumin < 1.2 g/dL is observed in exudates (87% of cases) (80)
Distinction of pleural fluid into di- or exudate
Identification of the type of pleural fluid, i.e. whether it is a di- or exudate, is essential in order to start the differential diagnosis process. Bidroma is usually caused by an imbalance of hydrostatic pressure and intravascular volume and typical causes of its formation are heart failure and liver cirrhosis. In most cases, the diastroma is reduced by proper treatment of the primary disease. Similarly, exudate is mainly formed when local intrathoracic factors prevent drainage of pleural fluid, in cases such as pneumonia, fever, malignancies and thromboembolism. Several criteria have therefore been proposed to objectively distinguish the type of pleural effusion. The best known and widely applied are Light's modified criteria (66):
- Pleural fluid protein/serum protein ratio > 0.5
- Pleural fluid LDH/serum LDH ratio > 0.6
- Pleural fluid LDH > 2/3 of the upper normal value of blood serum LDH
- Pleural fluid protein > 3 g/dL (29 g/L)
One criterion is sufficient to confirm that the type of liquid is an exudate
Similarly, other alternative criteria have been proposed to achieve the same distinction, such as (80):
- Pleural fluid LDH > 45% of the upper normal value of blood serum LDH
- Pleural fluid cholesterol > 45 mg/dL (1.16 mmol/L)
- Pleural fluid protein > 2.9 g/dL (29 g/L)
- The difference of serum protein minus pleural fluid protein < 1 g/dL (31 g/L)
- The difference between serum blood albumin minus pleural fluid albumin < 2 g/dL (12 g/L)
One criterion is sufficient to confirm that the type of liquid is an exudate
Several studies have been carried out to compare the above criteria. Most notably, Table 1 shows the superiority of Light's criteria over the others (81). Other studies confirm that the protein, lactate dehydrogenase (LDH) and cholesterol criteria have a 90% sensitivity rate in identifying pleural fluid as exudative (82).
Πίνακας 1
Diagnostic characteristics of pleural fluid markers
Causes of diphtheria pleural effusion
The most common causes of bifidromatic pleural effusion include (66):
- Left heart failure
- Cirrhosis of the liver
Less common causes include (66):
- Hypoalbuminemia
- Peritoneal dialysis
- Hypothyroidism
- Nephrotic syndrome
- Mitral valve stenosis
Finally, rare causes include (66):
- Compressive pericarditis
- Urinothorax
- Meigs syndrome
Causes of exudative pleural effusion
The most common causes of exudative pleural effusion include (66):
- Malignancies
- Parapneumonic collections
- Tuberculosis
- Empyema
Less common causes include (66):
- Pulmonary embolism
- Rheumatoid arthritis and other autoimmune pleuritis
- Benign asbestos pleural effusion
- Pancreatitis
- After a myocardial infarction
- After coronary heart surgery
Finally, rare causes include (66):
- Yellow-vein syndrome and other diseases of the lymphatic system
- Pharmacotherapy-induced pleural effusion
- Mycotic
Additional diagnostic tools in the investigation of pleural effusion
Transthoracic ultrasound
- Can easily show the existence of free or encapsulated pleural effusion (septum)
Ultrasound shows higher specificity but lower sensitivity, compared with chest CT with contrast, in the diagnosis of malignant pleural effusion, as shown in Table 2 (83).
Πίνακας 2
Diagnostic characteristics of imaging techniques
CT scan of the chest with contrast
- It should be performed before thoracentesis to highlight benign or malignant pleural thickening (66)
- It should be performed for complicated, inflamed, pleural effusion when drainage by tube is unsuccessful and surgery is likely to follow (66)
- Should be performed in patients with undiagnosed, exudative, pleural effusion to diagnose the cause (66)
Calibration of the probability of malignant pleural effusion based on CT scan
- A scoring system with a sensitivity of 74% and specificity of 92% has been proposed to determine whether a pleural effusion is malignant or benign according to the following chest CT imaging criteria (84):
- 5 points for the presence of pleural nodule or mass or thickening > 1cm
- 3 points for the presence of liver metastases
- 3 points for the presence of an intra-abdominal mass
- 3 points for the presence of an intrapulmonary mass or nodule > 1cm
- 2 points for the absence of pleural effusions
- 2 points for the absence of pericardial effusion
- 2 points for the absence of an enlarged cardiac silhouette
Total points > 7 confirms malignant pleural effusion
Thoracoscopy under local anaesthesia
- It should be performed when malignant pleural effusion is suspected and thoracocentesis is ineffective (66)
- Recommended for patients in good general condition and suspected malignant pleural effusion (85)
- Thoracoscopic pleural biopsy is considered one of the most effective tests for detecting a positive culture of mycobacterium mycobacterium (66)
- Can be used diagnostically and therapeutically (86)
- It is a safe procedure with a mortality rate of 0.34%, major complications 1.8% and minor complications 7.3% (86)
- May help in the differential diagnosis of undiagnosed pleural effusions (87)
- Thoracoscopy with rigid instruments appears to be superior to that with semi-rigid instruments in demonstrating the etiology of pleural effusion (88)
Percutaneous pleural biopsy
- It is indicated in patients with undiagnosed pleural effusion and suspected malignancy, when nodular formations are found on CT scan (66)
- Abrams needle biopsy may be useful in areas with an increased incidence of TB (66)
- Cutting needle biopsy under thoracoscopic or imaging guidance shows greater efficacy than Abrams needle biopsy in patients with suspected malignant pleural effusion (89)
- In patients with suspected malignant pleurisy, to improve diagnostic accuracy, a culture and histological examination of the specimens should be sent along with the culture and histological examination (66)
- Abrams needle biopsy under CT imaging guidance can approach the results of thoracoscopic biopsy (90)
- Cutting needle biopsy guided by transthoracic ultrasound may be useful in determining the cause of pleural effusion (91)
Bronchoscopy
- Not indicated as a routine test for the investigation of an undiagnosed pleural effusion (66)
- Relevant in patients with haemoptysis or suspected endobronchial obstruction (66)
Management of patients with pleural effusion
Figure 3
Diagnostic algorithm for patients with pleural effusion (92)
Modified by: Fig. 3 McGrath EE1, Anderson PB.
Diagnosis of pleural effusion: a systematic approach.
Am J Crit Care. 2011 Mar;20(2):119-27
Initial management begins with thoracocentesis and/or placement of a drainage tube to drain the pleural effusion and relieve the patient of symptoms such as respiratory distress (64). Figure 3 above shows one of the relevant algorithms.
Management of patients with benign pleural effusion
In patients with benign pleural effusion, after palliative drainage of the effusion, treatment focuses on treating the underlying disease that is secondary to the pleural effusion (66). When treatment of the primary disease cannot control the recurrence of the pleural effusion, pleurodesis is required. Typically, the iodinated povidone iodide technique has a success rate of 89% and is a good therapeutic approach for patients with recurrent, benign, pleural effusions (93). The presence of bilateral pleural effusion is associated with higher mortality compared with unilateral effusion (94). A proportion of 8-12% of patients with benign pleural effusion may develop malignancy in the first three years after the first appearance of the effusion (95)(96). The relevant algorithm for management follows below depending on the clinical suspicion (97):
- postoperative pleural effusion (figure 4)
- parapneumonic collection (figure 5)
- tuberculous pleurisy (figure 6)
- chylothorax and haemothorax (figure 7)
- haemothorax (figure 8)
Figure 4
Diagnostic algorithm for patients with postoperative pleural effusion (97)
Modified by: Fig.6 Villena Garrido et al
Recommendations of diagnosis and treatment of pleural effusion. Update.
Arch Bronconeumol. 2014 Jun;50(6):235-49. doi: 10.1016/j.arbres.2014.01.016. Epub 2014 Mar 31
Figure 5
Diagnostic algorithm for patients with parapneumonic effusion (97)
Modified by: Fig. 3 Villena Garrido et al
Recommendations of diagnosis and treatment of pleural effusion. Update.
Arch Bronconeumol. 2014 Jun;50(6):235-49. doi: 10.1016/j.arbres.2014.01.016. Epub 2014 Mar 31
Figure 6
Diagnostic algorithm for patients with tuberculous pleurisy (97)
Modified by: Fig. 4 Villena Garrido et al
Recommendations of diagnosis and treatment of pleural effusion. Update.
Arch Bronconeumol. 2014 Jun;50(6):235-49. doi: 10.1016/j.arbres.2014.01.016. Epub 2014 Mar 31
Figure 7
Diagnostic algorithm for patients with chylothorax (97)
Modified by: Fig. 7 Villena Garrido et al
Recommendations of diagnosis and treatment of pleural effusion. Update.
Arch Bronconeumol. 2014 Jun;50(6):235-49. doi: 10.1016/j.arbres.2014.01.016. Epub 2014 Mar 31
Figure 8
Diagnostic algorithm for patients with haemothorax (97)
Modified by: Fig. 8 Villena Garrido et al
Recommendations of diagnosis and treatment of pleural effusion. Update.
Arch Bronconeumol. 2014 Jun;50(6):235-49. doi: 10.1016/j.arbres.2014.01.016. Epub 2014 Mar 31

You can arrange an appointment with the doctor
In the morning the Thoracic Surgeon, Dr Athanasios Kleontas MD is at the Interbalkan Medical Center of Thessaloniki, while in the evening he is at his private office (73, Ermou St).
+30 2310 - 400000
Office 11, 2nd floor. Dec 1742
ATHANASIOS D. KLEONTAS
PATIENT VISITATION HOURS
Doctor is available (by mobile) 24 hours a day, 7 days a week.
You meet him only by appointment at his private office:
Monday to Friday : 18.00 - 21.00
