Intrathoracic hyperthermic chemotherapy (HITHOC)
Pleurodesis with periodic hyperthermic chemotherapy
(The text is taken from the doctoral thesis of Dr Athanasios Kleontas)
The action of hyperthermia at the cellular level
Increase in cell temperature in the range of 39-45 oC, can cause inhibition of their proliferation and cell death. The intensity of the effects depends on the combination of the temperature level and the time of exposure of the cells to that temperature, which together are expressed as thermal dose (111). Many changes occur during exposure of cells to elevated temperature. Temperature changes the characteristics of the cell membrane, causing modification of the cell morphology, intracellular concentrations of potassium and sodium ions and membrane potential (112-113). Surprisingly, none of the aforementioned phenomena directly correlate with impending cell death and therefore none of them adequately explain the direct mechanism of thermally induced cytotoxicity (112-113). Beyond membranes, secondary protein structures appear to be more sensitive to heat and perhaps heat-induced protein denaturation may explain the effects of mild hyperthermia on cells (111). Although DNA is not destroyed at temperatures of 39-45 oC, however, de novo DNA synthesis and polymerization exhibit sensitivity to heat, due to induced denaturation and aggregation of synthetases and polymerases (114-115). This appears to contribute significantly to cell cycle and cell death functions. It also causes disruption of key enzymatic functions of the cell, such as the DNA repair process. Nuclei are sensitive to hyperthermia, as are other enzymes used in RNA synthesis. Furthermore, there is an inability of RNA to interact with proteins during hyperthermia (116). Finally, after a sufficient thermal dose, cells are driven to death either through necrosis, where the cell very rapidly loses the integrity of its cell membrane, or through apoptosis, where programmed cell death is hastily triggered. Each of these two processes is subject to different immunoregulatory activities (117). Figure 12 below highlights the main biological ways in which the effect of hyperthermia attempts to produce therapeutic effects through the following immunological effects (118):
- Expression of MICA, MHC(I) molecules on the surface of tumor cells subjected to hyperthermia
- Heat shock protein production
- Production of exosomes
- Direct effects on immune cells
- Modification of the vascularization of tumors
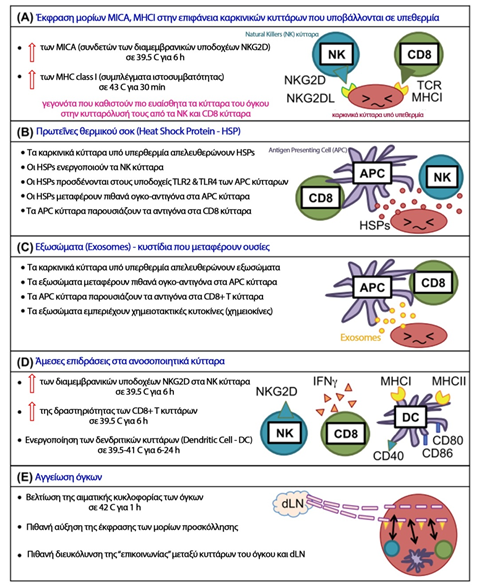
Figure 12
Hyperthermia-induced immune activation mechanisms (118)
Modified from: Fig.1 Local tumour hyperthermia as immunotherapy for metastatic cancer
Seiko Toraya-Brown1 & Steven Fiering
Int J Hyperthermia, 2014; 30(8): 531–539
The application of hyperthermia for therapeutic purposes
Hyperthermia, as a therapeutic process, is performed by increasing the temperature of a specific anatomical area or the whole body to levels above the normal temperature, in order to induce therapeutic effects in the body. There are three different basic categories of hyperthermia application (118):
- Local hyperthermia
- Regional hyperthermia
- Whole-body hyperthermia
In the procedure of local hyperthermia, usually for the treatment of neoplasms, the temperature increase is applied to a solid tumour and may reach 80oC when attempting to resect (destroy) the tumour (119) or may range between 41-45oC when the aim is to cause only severe damage to the physiology of the cells, leading to cell death, without causing serious injury to neighbouring healthy tissues (120).
In periodic hyperthermia, the temperature increase is applied to a relatively large anatomical area of the body, such as the peritoneal cavity, pleural cavity, upper or lower limb, and the temperature of the selected area is kept between 39-42 oC so that the benefit is derived from physical effects similar to those caused by fever, without causing tissue damage (118).
In whole-body hyperthermia, the whole body is immersed in a warm solution or wrapped in thermal blankets to achieve hyperthermia at a temperature between 39-41 oC. Whole-body hyperthermia is usually combined with other techniques in extensive, metastatic malignancies (121).
Hyperthermia for the treatment of neoplasms
Hypertermia is therapeutically applied to treat neoplasms, as the natural response of normal cells differs from that of neoplasmic cells to the effect of hypertermia. It is known that the increase in temperature in a tissue causes an immediate and corresponding increase in blood flow, which is accompanied by vascular dilation and increased permeability of the vessel wall, as shown in Figure 1. (122). Compared to healthy tissues, neoplasms appear to show a decreased ability to increase blood flow as the temperature rises. Consequently, the diffusion of heat through the blood flow in the tumors is slower than the corresponding in normal tissues, resulting in tumors to develop a higher temperature than the normal tissue when they are heated (higher temperature causes greater damage), since the decrease in blood flow and therefore the delay in the removal of blood from the tumor with venous circulation, also decreases the rate of return of the temperature of the neoplastic tissue (heat clearance) to normal levels. (123). This is related to the structural differences of the vascular network of the tumors, where the newly formed vessels in a neoplasm are created by pre-existing vases, morphologically simulate with hypertrophic capillaries and are characterized by a partial or even complete absence of smooth muscle fibers and pericles. Due to the fact that the blood flow in the tumors depends on their size and their type (larger blood flow is observed in small tumors), several times greater flow to the tumours from the surrounding healthy tissues is noted. Also the vascular network of tumors can be damaged at certain temperatures, while at the same temperatures the vasculary network of normal tissues show only minimal lesions (122).
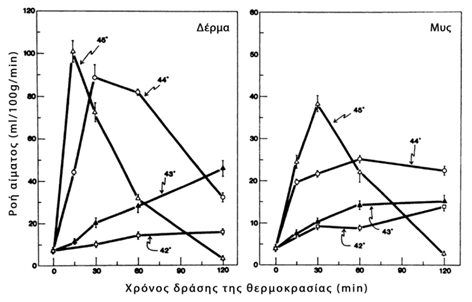
Graph 1
Effect of temperature on blood flow in tissues (122)
Modified from: Fig. 1 Effect of local hyperthermia on blood flow and microenvironment: a review.
Song CW
Cancer Res. 1984 Oct;44(10 Suppl):4721s-4730s.
Initially there is an increase in blood flow due to vasodilation and after a period of time - which depends on the type of neoplasm and the temperature level - there is a reduction in blood flow, which is due to endothelial thickening and microthrombosis. This phenomenon is more pronounced when the applied temperature exceeds 42°C and may play a role in the hyperthermia-chemotherapy combination, considering that the chemotherapeutic drug once it reaches the neoplasm, due to the subsequent reduction in blood perfusion of the tumour, will remain and affect the tumour for a longer period of time (124).
Comparative studies on cultures of human normal and neoplastic cells, exposed to temperatures of 42.5-43.0°C for 4-8 h, showed higher rates of neoplastic cell necrosis with statistical significance (p<0.01), as shown in Figure 2 below (125).
It was also observed that although neoplastic cells have increased mitotic activity, (but less than some normal cells, such as uveal melanocytes) this is not associated with increased temperature sensitivity.
Regional chemotherapy
Regional chemotherapy is based on the idea of improving the efficacy of the chemotherapeutic agent on the malignancy when administered close to the anatomical area where the tumour is located. Compared to systemic chemotherapy, when performing regional chemotherapy, it is possible to administer high doses of chemotherapeutic drugs in a short time and achieve high tissue concentrations in the tumour site, while limiting undesirable, systemic side effects (126).

Graph 2
Effect of temperature on survival
healthy and neoplastic cells (125)
Modified from: Fig. 1-4 Selective lethal effect of supranormal temperatures on human neoplastic cells.
Giovanella BC, Stehlin JS Jr, Morgan AC.
Cancer Res. 1976 Nov;36(11 Pt 1):3944-50.
The main differences between systemic and regional chemotherapy are discussed as follows (127-129):
- With systemic administration, the drug is distributed throughout the body, whereas with regional administration it is restricted to one organ or anatomical area (e.g. liver, upper or lower limb, peritoneal or pleural cavity).
- In systemic administration, an amount of the chemotherapeutic drug (up to 70% in some cases) is metabolised and degraded (mainly in the liver) before reaching the final target organ, whereas in intermittent administration, almost all of the drug acts on the tumour (expecting higher response rates to treatment) and only a small percentage enters the systemic circulation.
- Periodic chemotherapy achieves continuous and prolonged exposure of the neoplastic cells to the chemotherapeutic agent. Periodic administration is characterised by much less or no systemic toxicity. In addition, regional chemotherapy allows the administration of much higher doses (which in systemic administration are considered prohibitive due to their toxicity), with the main objective of increasing the concentration of chemotherapeutic agents to overcome the problem of tumour resistance.
5.3.4.1 Methods of administration of regional chemotherapy
There are two main categories of peripheral chemotherapy (126):
Intravascular administration, which can be divided into:
- Pure intra-arterial infusion - in primary or metastatic tumours
- Combination of intra-arterial infusion with chemoembolization - in liver tumours
- Intra-arterial injection - Isolation Perfusion - in sarcomas and melanomas of the extremities
- Intra-arterial infusion with synchronous vascular blockade and hypoxemia (Stop flow) - in lesser pelvic tumours
Intra-abdominal administration , which is applied to three different anatomical sites: the peritoneal cavity, the pleural cavity and intracystic (bladder).
When administered intravascularly, it is used as an access vessel either:
- the main feeding vessel of the organ or neoplasm (which is not necessarily the same). A typical example is the hepatic artery in the liver, because it is the one that mainly irrigates macroscopic metastatic foci, although it supplies only 14% of the total parenchymal hepatic flow. In contrast, normal liver cells, and thus microscopic metastatic foci, which have not yet acquired an independent perfusion, are mainly perfused by the portal vein (130-131).
- either the arterial trunk supplying the anatomical region in which the target organ or neoplasm is located (catheterisation of the external iliac vessels for neoplasms of the lower limbs using the Isolation Perfusion method, Stop-flow technique for neoplasms of the lesser pelvis with transient occlusion of the abdominal artery, etc.).
Figure 13 below highlights the mode of intravascular, peripheral lower extremity chemotherapy for the treatment of lower extremity melanoma (132).

Figure 13
Regional lower limb chemotherapy (132)
Modified from: Fig. 2 Management of In-Transit Malignant Melanoma
Paul J. Speicher, Douglas S. Tyler and Paul J. Mosca
ISBN 978-953-51-0961-7
Intra-abdominal administration of chemotherapeutic drugs mainly involves the peritoneal and pleural cavity. The homogeneous distribution of the chemotherapeutic solution is naturally limited by the presence of adhesions between the intestinal helices or between the helices and the abdominal wall in the case of the peritoneal cavity and between the lung and the chest wall, mediastinum and diaphragm in the case of the pleural cavity. In the following figure 14, regional intrathoracic chemotherapy with thoracoscopic placement of the circuit tubes is distinguished (133).
Patients with primary pleural tumours, metastatic pleural tumours and metastatic malignant pleural effusion usually have an unfavourable prognosis, with a median survival rate ranging between 6-18 months. Therefore, the combination of cytoreductive (oncemic), surgical intervention with regional, intravesical chemotherapy followed by systemic chemotherapy is increasingly used for local disease control (1, 104).

Figure 14
Regional pleural cavity chemotherapy (133)
Modified from: Fig.2 Clinical outcomes of cytoreductive surgery combined with intrapleural perfusion of hyperthermic chemotherapy in advanced lung adenocarcinoma with pleural dissemination
Eunjue Yi , Daejoong Kim , Sukki Cho, Kwhanmien Kim, Sanghoon Jheon
J Thorac Dis. 2016 Jul; 8(7): 1550–1560.
Combination of regional hyperthermia and chemotherapy
Classical studies have demonstrated the remarkable, synergistic effect of hyperthermia and chemotherapy. An oversimplified explanation of this synergy is that: although chemotherapy causes severe damage to the neoplastic cells, they retain their ability to repair the damage caused - at this point hyperthermia acts synergistically, inhibiting the repair mechanisms, leading to the death of the neoplastic cells. Indeed, the term 'thermochemotherapy' is attributed to this synergistic action (134). Figure 3 below shows that the induced mortality of neoplastic cells increases with the synergistic action of hyperthermia and chemotherapy and is proportional to the temperature and concentration of the chemotherapeutic agent (134).
Also, other research studies have highlighted the increased cytotoxicity of certain chemotherapeutic agents (such as docetaxel, paclitaxel, irinotecan, oxaliplatin, gemcitabine) when they act under mild hyperthermia (41.5oC) conditions due to increased tumour penetration (135). Therefore, in the last 20 years, intraoperative, regional, intraperitoneal, hyperthermic chemotherapy following oncemic surgery has been performed in cases of malignant mesothelioma, peritoneal pseudomyxoma and peritoneal disseminated colorectal, gastric and ovarian cancers, with very good results, in terms of overall survival, disease-free interval and topoperative control of the disease (136-140).
In contrast, intraoperative, regional, intrathoracic (intrathoracic or intralesional), hyperthermic chemotherapy is not as widely used and applied, and studies addressing this issue are limited (4, 141). The first relevant publication was made in 1995 by Matsuzaki et al (142) and since then about 30 articles have been published (4).
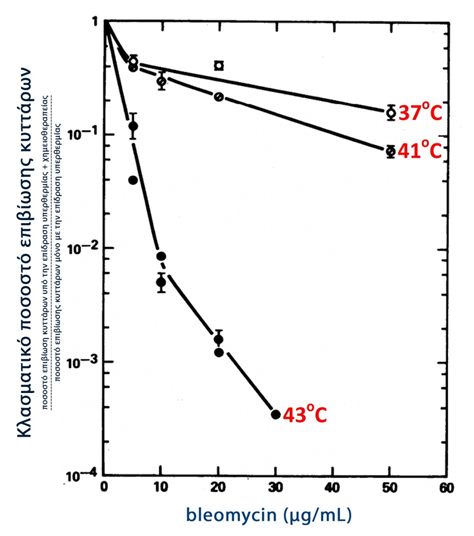
Graph 3
Survival of neoplastic cells under the synergistic effect
chemotherapy and hyperthermia (134)
Modified from: Fig.1 Thermochemotherapy: synergism between hyperthermia (42-43 degrees) and adriamycin (of bleomycin) in mammalian cell inactivation.
Hahn GM, Braun J, Har-Kedar I.
Proc Natl Acad Sci U S A. 1975 Mar;72(3):937-40.
Hyperthermic intrathoracic chemotherapy in the treatment of malignant pleural effusion
Hyperthermic Intrathoracic Chemotherapy (HITHOC - or Intrapleural Perfusion ThermoChemotherapy - IPTC) started and continues to be used mainly intraoperatively after cytoreductive surgery, when it is not possible to achieve R0 (radical) resection of tumours with a primary focus in the lung, pleura and thymus or metastatic tumours with spread to the pleural cavity, originating from the breast and ovaries(4-6, 143). Table 6 below shows the five unique comparative studies in the literature, whose meta-analysis demonstrates the statistically significant superiority (p<0.001) of the HITHOC technique in terms of median survival, survival rate at year 1, disease-free period and Karnofsky scale (4).
In particular, in the treatment of thymic carcinomas with concomitant malignant pleural effusion, the results of HITHOC in combination with cytoreductive surgery showed a median survival of 18-54 months (144-146). The superiority of HITHOC was also concluded in a meta-analysis of studies in which the HITHOC technique was applied, again in combination with cytoreductive surgery, in patients with malignant pleural mesothelioma (147).
In metastatic, malignant, pleural effusion from non-small cell lung cancer, the HITHOC technique has been applied thoracoscopically (VATS) with great success, without major complications and a mean survival of 21.7 months, a survival rate at year 1 of 74.1% and finally an increase in performance status of 89.3%. This makes the HITHOC-VATS (IPTC-VATS) technique a new, safe, less invasive and more effective method to treat malignant pleural effusion in lung cancer patients (148).
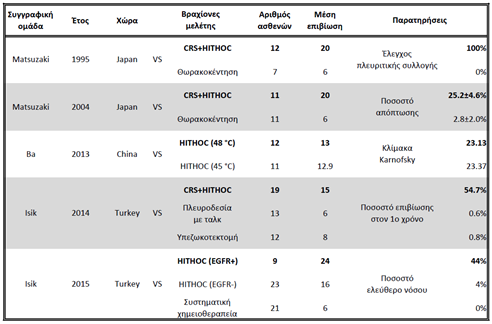
CRS: CytoReductive Surgery
HITHOC: Hyperthermic IntraTHOracic Chemotherapy
EGFR: Epithermal Growth Factor Receptor
Πίνακας 6: Meta-analysis of comparative studies where it was applied HITHOC
The chemotherapeutic agents that have been used in the HITHOC technique are cisplatin, doxorubicin and mitomycin C at temperatures of 41-43oC (4). In fact, it has been estimated that cisplatin with the aid of hyperthermia can penetrate up to 3-4 mm deep from the free surface of the lung parenchyma (149). The combination of two chemotherapeutics seems to be superior to one, without being validated by large studies (150).
The application of HITHOC causes a temporary dysfunction in the microcirculation of the tissue structures where it is applied, which is gradually restored after 72 hours (151). The main complications that have been reported after HITHOC are fever, bleeding, air escape, atrial fibrillation, pulmonary embolism, chest pain, fever, dyspnoea, bronchopulmonary fistula, in very low rates, and mild cardiotoxicity has been observed only in the case of co-administration of two chemotherapeutic agents (cisplatin + doxorubicin) (4, 152). The mortality associated with the technique is zero, even when the technique is repeated five times in the same patient, as is the case in a large centre in China, where the technique is performed under local anaesthesia at the patient's bedside (153).
In the last 10 years, due to the excellent results of the method in prolonging survival and improving patients' quality of life, there are 17 thoracic surgery departments in Germany that use the method and have already performed more than 350 procedures (154).
In our country the method has been accepted by the Ministry of Health in 2011 and is applied in limited centres (No.Prot:Y4a/oik.131099, 29.11.2011).
According to the results of the PhD thesis of Thoracic Surgeon Athanasios Kleontas, as announced in a prestigious international scientific journal in 2019, intrathoracic hyperthermic chemotherapy is a good and safe therapeutic option in the treatment of patients with malignant pleural effusion in non-small cell lung cancer, with acceptable survival.

You can arrange an appointment with the doctor
In the morning the Thoracic Surgeon, Dr Athanasios Kleontas MD is at the Interbalkan Medical Center of Thessaloniki, while in the evening he is at his private office (73, Ermou St).
+30 2310 - 400000
Office 11, 2nd floor. Dec 1742
ATHANASIOS D. KLEONTAS
PATIENT VISITATION HOURS
Doctor is available (by mobile) 24 hours a day, 7 days a week.
You meet him only by appointment at his private office:
Monday to Friday : 18.00 - 21.00
